The Qbitus Mercury 300 from MMS Medical is designed for high risk users, with particular emphasis on the I.T. area and possibly needing some degree of postural control without inhibiting the transferral process.
QBITUS MERCURY 300
Specification
- Sizes Up: to 20″ X 18″. All cushions are 3½” in thickness. Larger sizes are available.
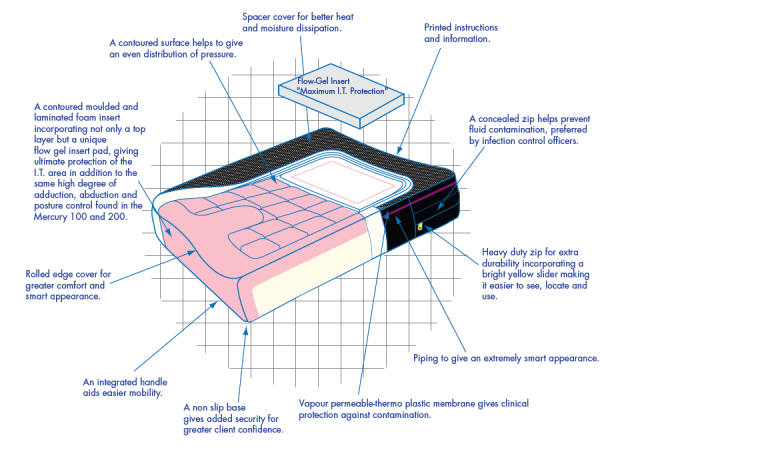
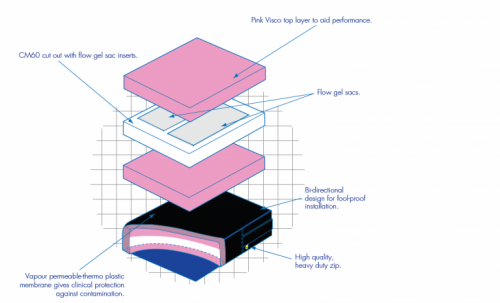
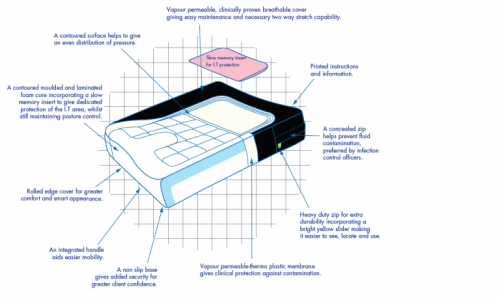
- Cover: A Spacer cover is issued as standard equipment, chosen for its superior air circulation and comfort, trimmed with piping for a smart appearance. The side banding is constructed using vapour permeable material on a non slip base for added security. It incorporates a high quality zip wwith a highly visible yellow slider for easy location and usage, concealed for increased clinical performance. A vapour permeable thermo plastic interliner is included as standard.
- Risk Category *: High Risk with posture Control
- Foam Insert: The Mercury 300 is constructed using a multi-layer format with each material selected for its particular properties : the cold cured foam base for its high resilience, durability and support: a slow memory overlay for reduction in interface pressure and protection and particular attention paid to the I.T. area with the inclusion of a flow gel sac or high performance slow memory foam manufactured specifically for this application. All layers are integrated into a low profile product with built in adduction and abduction.
- Weight Guide **: For maximum comfort, durability and performance the weight guide on a 17″ X 17″ cushion is 17-18 stone ( 108 – 114kg)
Support Pressure 1lb per square inch at 50% compression or deflection - Linear Load Limit: 31 stone ( 196kg) on a typical 17″ X 17″ cushion
*Risk categories are given as a guide only.
**Weight Guidelines are a general indication of an ideal user weight to give optimum cushion perfomance